When purchasing supplements and vitamins, consumers often encounter various labels and dates on the packaging. One such label is the “mfg date.” One commonly encountered term is “mfg,” short for manufacturing date. Understanding what this term means is crucial for manufacturers and consumers and ensuring the quality and effectiveness of dietary supplements. This article aims to clarify the significance of the “mfg date” and provide insights into its implications for both manufacturers and consumers.
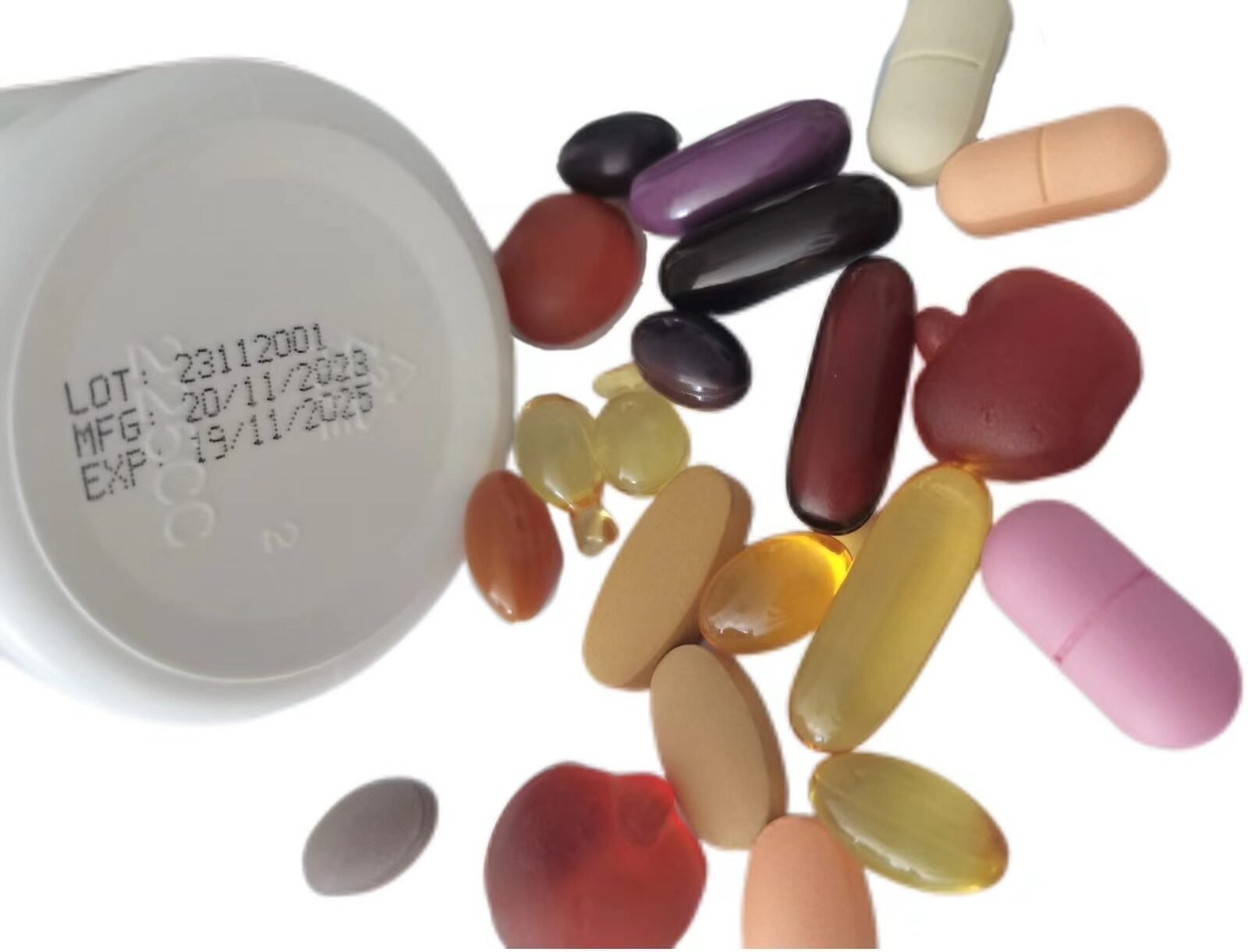
Mfg Date Meaning
‘Mfg’ date stands for the manufacturing date, is the date on which a vitamin or supplement product was produced. Previously, USP guidelines required supplement manufacturers to state an expiration date, ‘Best before’, ‘Use by’ or ‘Sell by’, date. The guidelines were revised by the FDA to include an ‘mfg’ date as well. It signifies when the product was made, encapsulated, or bottled, marking the beginning of its shelf life.
Importance of the Manufacturing Date
The manufacturing date is critical for several reasons:
Quality Assurance: It helps ensure the product was produced following good manufacturing practices (GMP).
Traceability: It aids in tracing the product’s production batch, which is essential in case of recalls or quality issues.
Difference Between “Mfg Date” and Expiry Date
It’s vital to distinguish between the manufacturing date and the expiry date:
Manufacturing Date: Indicates when the product was created.
Expiry Date: The date after which the product is no longer guaranteed to be effective or safe to use.
Stability and Potency of Supplements
The stability and potency of supplements can degrade over time. The “mfg date” helps consumers and manufacturers monitor:
Potency: The effectiveness of active ingredients. The effectiveness of active ingredients can diminish over time.
Stability: Environmental factors like temperature and humidity can affect the product’s stability.
Consumer Awareness and Education
Educating consumers about the “mfg date” can lead to better purchasing decisions:
Reading Labels: Encouraging consumers to check the manufacturing date before purchasing.
Understanding Freshness: Helping consumers know the importance of fresher supplements for optimal efficacy.
Regulatory Requirements and Compliance
The dietary supplement industry is regulated to ensure product safety and quality:
Labeling Regulations: Manufacturers must adhere to strict guidelines for displaying manufacturing dates.
Quality Control: Adherence to GMP standards and regular quality checks.
Industry Best Practices
Manufacturers can adopt best practices to ensure high-quality supplements:
Batch Testing: Regular testing of production batches for potency and contamination.
Transparent Labeling: Clearly displaying manufacturing dates and other critical information on labels.
Common Misconceptions
Clearing up common misconceptions about “mfg dates”:
Confusing “mfg date” with Expiry Date: The manufacturing date is not the same as the expiry date; it’s the start point of the product’s shelf life.

Conclusion: Making Informed Choices
Understanding the “mfg date” on supplements and vitamins is vital for making informed choices. By paying attention to this date, consumers can ensure they are purchasing fresh and effective products, while manufacturers can maintain high standards of quality and compliance.
This article aims to provide a clear and comprehensive understanding of the “mfg date,” helping both consumers and manufacturers navigate the complexities of vitamin and supplement packaging. educate consumers and help them make better purchasing decisions, while also emphasizing the importance of quality control and compliance for manufacturers in the dietary supplement industry.



